Ammonia Hydroxide Acid Or Base
What is NH4OH (Ammonium Hydroxide)?
NH4OH is the chemic formula of a solution of ammonia in water. This solution is known past diverse names such as ammonia water, ammonium hydroxide, ammonia liquor, and aqueous ammonia. NH4OH is oftentimes denoted past the symbol 'NH3 (aq)'.
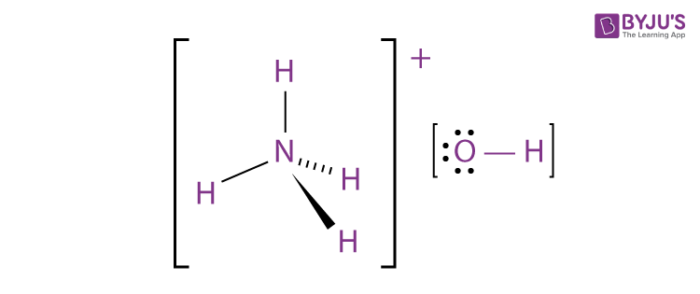
The general structure of an NH4OH molecule is illustrated above.
Basicity of NHfourOH
When ammonia is dissolved in water, the h2o molecules donate a proton to the NHiii molecule. This leads to the formation of an ammonium cation (whose chemical formula is NH4 +) and a hydroxide ion (OH–). This can be represented by the following equilibrium reaction.
NHthree + H2O ⇌ NH4 + + OH–
A solution of ammonia in water with a concentration of 1M displays a 0.42% conversion of ammonia into ammonium cation.
A solid base can ionize fully in the presence of H+. This means that in the presence of H+, OH– will exist completely ionized to form water and complementary salt from its counterpart. Let u.s. take the example of a solid base of NaOH dissolved in water. In the presence of H+ ions (from water OH– and H+), NaOH is readily formed by H2O and NaOH since the positively charged sodium interacts with negatively charged OH– from h2o, while Na+ interacts with OH- from Na+OH–.
The production would be NH4 +OH– + H2O in the case of ammonium hydroxide. Remember that the results of each reaction are the same equally the reactants.
Saturated Solutions of Ammonium Hydroxide
The solubility of ammonia decreases with the increment in temperature of the water solvent. Information technology can be observed that this behaviour of ammonia is quite similar to that of other gases. The solution of ammonia in water exhibits a decrease in its density with the increase in the concentration of dissolved ammonia.
The density of a saturated ammonium hydroxide solution at 288.75 1000 is equal to 0.88 grams per mole. This saturated solution of ammonium hydroxide would contain approximately 35.6% ammonia by mass which corresponds to 308 grams of ammonia per litre of saturated ammonium hydroxide solution.
Backdrop of Ammonium Hydroxide (NH4OH)
An NHfourOH solution has the following properties in its standard state:
- NH4OH has a molar mass of 35.04 grams per mole.
- It has a colourless appearance in its liquid country.
- Ammonium hydroxide solutions accept a highly pungent, "fishy" aroma.
- It has a density of 0.91 grams per cubic centimetre for a 25% westward/w solution. A 35% west/w solution would have a density of 0.88 grams per cubic centimetre.
- Ammonium hydroxide solution with a 25% mass fraction has a melting point of 215.seven K and a boiling point of 310.eight K
- Its standard enthalpy of formation is -80 Kilojoules per mole.
Uses of Ammonium Hydroxide
Ammonia water has many applications in industry as well as in laboratories. V such uses of ammonia solutions are listed below.
- Ammonium hydroxide solution is an important tool in the manufacturing procedure of chemical fertilizers. Information technology is used equally a solution or as table salt in these fertilizers.
- Ammonium hydroxide is besides used in the production of organic and inorganic chemicals containing nitrogen. Information technology is the base chemical in the manufacture of nitric acrid.
- A 1-3% solution of NH4OH is used in cleaning agents such equally window cleaning liquids. A solution of ammonia in h2o (scented or manifestly) is likewise sold directly as a cleaning amanuensis.
- Ammonia tin can be used in the production of chloramine which is a good disinfectant. It remains active in nevertheless water for longer durations than chlorine.
- Any wood that contains tannic acid can be sealed in a container with ammonium hydroxide solution to give a nighttime stained look to the woods. Thus, the ammonium hydroxide solution is used in furniture concealment.
Ammonium Hydroxide Hazards
Ammonium Hydroxide is extremely unsafe in case of touch with the skin (corrosive, irritant, permeator), eye contact (irritant), swallowing, etc. The eyes are non-corrosive. Non-corrosive to the lungs. Liquid or spray mist tin cause tissue damage, especially to the mucous membranes of the optics, mouth and respiratory tract.
Ammonium Hydroxide in contact with the pare can cause burns. Inhalation of the spray mist can crusade extreme inflammation of the respiratory tract, characterized past coughing, choking, or shortness of breath. Extreme over-exposure will atomic number 82 to death. Inflammation of the optics is marked by redness, swelling and scratching. Inflammation of the skin is marked by swelling, scaling, reddening or, rarely, blistering.
In instance of skin touch, quickly wash the peel with plenty of water for at least 15 minutes while removing dirty clothes and shoes. Fill up with an emollient on the irritated face. Hot h2o should exist used to disinfect clothing before reuse. Clean your feet advisedly earlier you supercede them. Get medical attention immediately.
As soon as possible, evacuate the patient to a secure place. Lose the plumbing fixtures apparel, such as a shirt, a scarf, a belt or a waistband. If ventilation is difficult, use oxygen. If the patient is non live, make a oral fissure-to-rima oris resuscitation. This can be dangerous for a person delivering oral-to-mouth resuscitation assistance where the inhaled substance is poisonous, contagious or corrosive. Get medical attention.
Frequently Asked Questions – FAQs
What is NH4OH in chemistry?
Ammonium hydroxide (NHfourOH) is an ammonia solution of water. Commonly referred to as ammonia or ammonia gas, the compound is used equally a cleaner and in the manufacturing of plastics, rubber, fertilizers and textiles. Information technology'due south a colourless, white liquid with a pung-to-faint ammonia scent.
Is ammonium hydroxide harmful to humans?
Ammonium hydroxide causes irritation of the pare; contact can cause astringent irritation and burns. Ingestion can cause diarrhoea, nausea, gastric hurting and, in farthermost cases, perforation, fundamental nervous system agitation, daze, seizures and pulmonary oedema.
What is the difference between ammonia and ammonium hydroxide?
Ammonium hydroxide is household ammonium in water. 1 of their main distinctions is that ammonia has no water content whereas ammonia hydroxide has water. This means that ammonium hydroxide is an ammonia solution with water content. It has merely a small amount of ammonia and its formula is NH3 (aq).
Is ammonium hydroxide corrosive?
Ammonia has alkaline properties and corrosive backdrop. Ammonia gas is readily dissolved in water to course ammonium hydroxide, a caustic solution with a heavy base of operations. Ammonia gas is easily compressed and under pressure forms a clear liquid. Ammonia is typically delivered in steel tanks as a solid material.
How do you fix ammonium hydroxide solution?
Preparation of nitrogen hydroxide. Ammonium hydroxide is formed by saturating the water with gaseous ammonia. Ammonia gas is generated and transmitted directly from the flask without the introduction of a drying tube into an empty gas washing canteen, which is used to collect whatsoever solid particles that are mechanically transported.
Why is it chosen aqueous ammonia ammonium hydroxide?
These methods of contrast both demonstrate that the solution contains more acidic ammonia (and water) molecules than ammonium and hydroxide ions, and these solutions are more specifically referred to as aqueous ammonia and not ammonium hydroxide.
What pH is ammonia solution?
Ane ammonia molecule consists of ane nitrogen ion that is negatively charged and iii hydrogen ions that are positively charged, giving ammonia a chemical NH3 formula. Normal ammonia has a pH of around eleven.
How strong is ammonium hydroxide?
A solid, pungent, suffocating odour of ammonium hydroxide is caused by the release of ammonia gas from the solution. Many ammonium hydroxide solutions range from less than i percent to around 35 pct ammonia in concentration.
To acquire more near ammonium hydroxide and other important chemic compounds, register with BYJU'S and download the mobile awarding on your smartphone.
Ammonia Hydroxide Acid Or Base,
Source: https://byjus.com/chemistry/nh4oh-ammonium-hydroxide/
Posted by: brannsonsise.blogspot.com



0 Response to "Ammonia Hydroxide Acid Or Base"
Post a Comment